Taking part in a clinical trial
1. Find a trial
![]()
Your doctor might suggest a clinical trial.
You can also search for clinical trials.
2. Eligibility
![]()
Most clinical trials specify the characteristics a person must have to join the trial. Your doctor will check if you meet the criteria to join.
3. Read and decide
![]()
Read the Participant Information Sheet and Consent Form (PICF). If you agree to join, you will be asked to sign the PICF.
4. During the trial
![]()
Follow instructions you are given about the trial and talk to the trial team about any symptoms you may experience.
5. After the trial
![]()
The trial team may stay in contact with you for follow up.
Learn more about each step of the process to take part in a clinical trial:
Steps to take part in a clinical trial
The treating doctor or the GP might suggest a clinical trial as an option. Family, friends or carers can also help find available clinical trials online.
It is important to learn about clinical trials from different sources. Look at general information and also specific clinical trials.
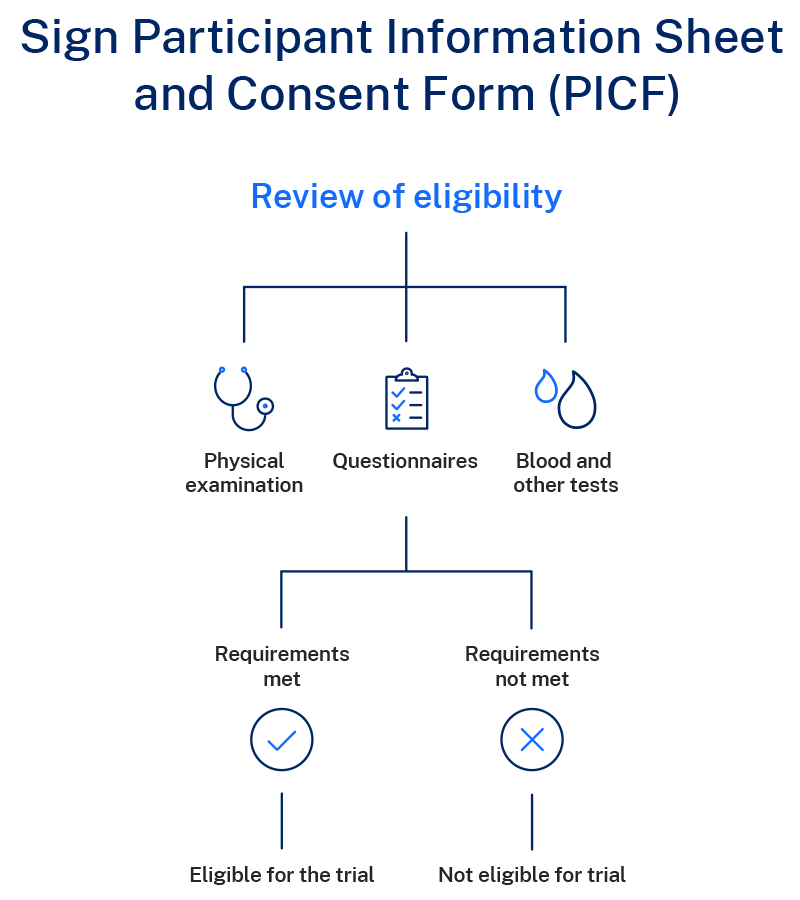
Determining if a trial is suitable for a person will depend on many factors, including the questions the trial is trying to answer. Most clinical trials have specific characteristics required for participation, for example, the type of cancer, age and how well the person is currently feeling.
The trial team will ask questions and request tests to check if a person can join the trial.
These guidelines are called eligibility criteria and it is specific for each trial. To become an eligible trial 'participant', a person must meet all inclusion criteria and have none of the characteristics listed in the exclusion criteria.
During the eligibility review, the trial doctor and the trial team will obtain the participant`s medical history and may ask for additional tests.
People who volunteer to join a clinical trial will be asked to sign a Participant Information Sheet and Consent Form (PICF). This document explains in plain language all aspects of trial participation, tests performed, visit schedule and duration of the trial. It also contains the contact details of the trial team, in case the participant needs to contact them.
The PICF ensures volunteers understand their involvement a trial, but it is not a legal contract.
Discussion with a treating doctor or health care team is encouraged. It is also recommended that family or carers be involved in the decision to enrol in a clinical trial or not.
The research team will look after all aspects of the trial and coordinate the care, including visits to the clinic, tests and consultations. It is important to follow the schedule as this will ensure the participant's safety during the trial.
A person can withdraw from a clinical trial at any time and still receive the best available treatment.
The trial team may still want to check on participants' wellbeing from time to time, either by phone or asking participants to visit the hospital. These follow up visits are important to collect additional information about the research and the participant's health.
I have cancer... is a clinical trial an option for me?
View our resource about clinical trials, also available in other languages.
You will find information about:
- what clinical trials are,
- how to decide if a trial is right for you,
- benefits and risks and,
- what's involved in participation.