Research Project Approval Process
Step 1: Lead Researcher submits sub-study protocol to Community of Practice
Sub-study protocols are submitted by the Lead Researcher on behalf of the research group. Protocols are due one week before the Community of Practice meeting and are submitted to the CanDLe Secretariat.
Step 2: Community of Practice reviews sub-study protocol
The Community of Practice members will review all sub-study protocols prior to the meeting. The Lead Researcher will provide a summary and justification of the proposed project at the meeting. The Community of Practice will have an opportunity to clarify any issues and provide any feedback to improve the project. Once the Community of Practice is happy with the sub-study protocol, it is recommended to the CanDLe Co-ordinating Principal Investigator for review by the ethics committee.
Step 3: Ethics Committee reviews sub-study protocol
The CanDLe team will submit the recommended sub-study protocol to the PHSREC on behalf of the CanDLe Co-ordinating Principal Investigator and inform the Lead Researcher of the outcome.
Step 4: Following approval from the ethics committee
All researchers accessing data must complete and return a copy of the Data Access Declaration. Once these have been returned, the CanDLe team will notify the secure data environment (SURE or ERICA) to organise access for the approved users. Mandatory training must be completed by all users before access to data is granted.
Step 5: Access to data is granted
Researchers may only access data after the following requirements have been met:
- The Authority to Disclose for the Lead Researcher has been signed off by Executive Director, Centre for Epidemiology and Evidence.
- The Lead Researcher has returned a signed Confidentiality undertaking (signed by the Institution’s delegate).
- The research team’s sub-study protocol has been approved by the PHSREC.
- All research team members accessing the data have returned a signed copy of the Data Access Declaration.
- All research team members accessing the data have completed the relevant secure data environment mandatory training.
- Any institutional/site governance requirements must be met prior to the commencement of any research by the Lead Researcher. Please check with your research office for confirmation of any requirements.
Step 6: Analysis of CanDLe data
All approved data users may analyse the data for the purpose of the approved sub-study protocol.
Step 7: Output Review
All outputs must be submitted to Cancer Institute NSW for review prior to submission for publication. Please send to: CINSW-CanDLeProgram@health.nsw.gov.au
Step 8: Annual Reporting
Ongoing ethical approval requires the submission of an annual progress report to the ethics committee. Lead Researchers must submit an annual progress report to the CanDLe secretariat for each sub-study protocol they are responsible for by 31 October each year. Please send to: CINSW-CanDLeProgram@health.nsw.gov.au
A final report must be submitted once the project has been completed. All outcomes and planned publications/presentations must be included in this report.
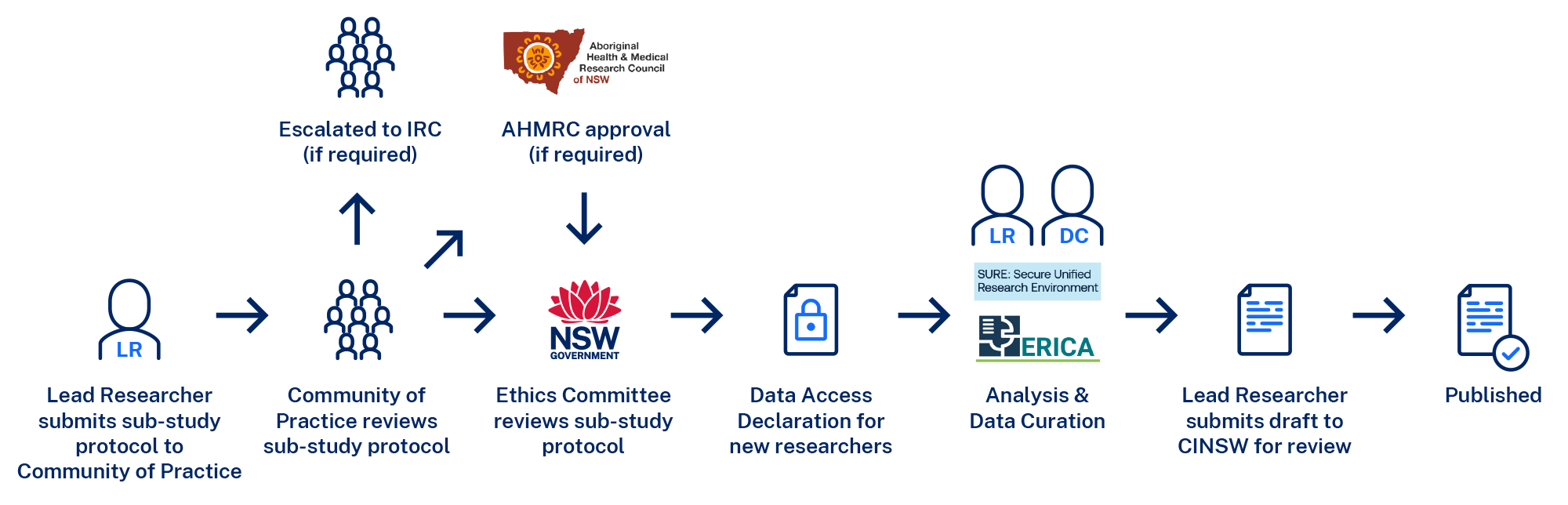
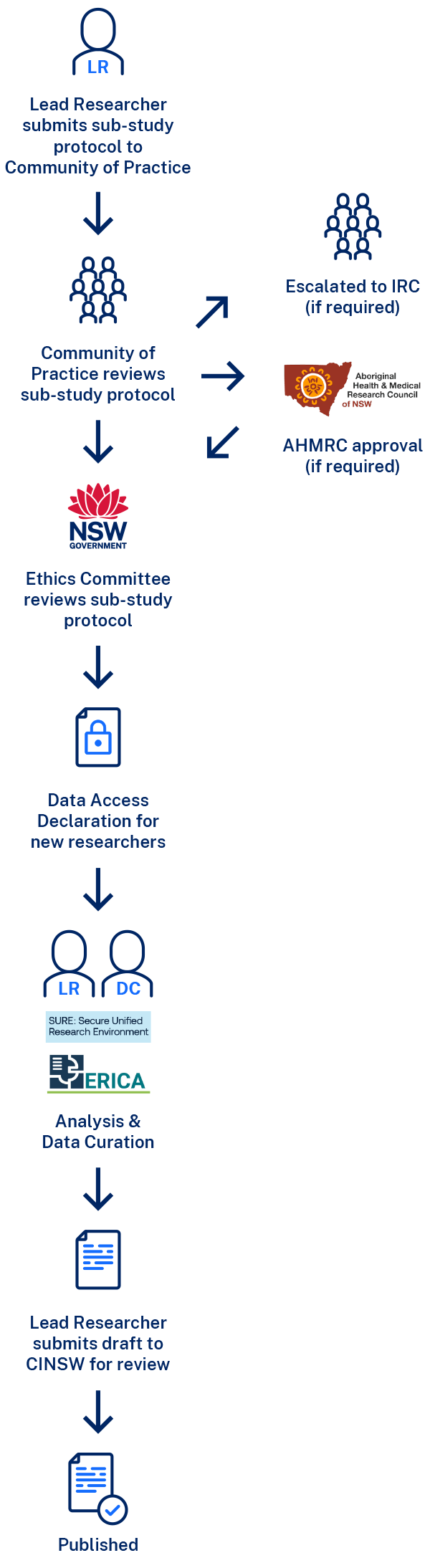
AHMRC = Aboriginal Health & Medical Research Council
ATD = Authority to Disclose
CoP = Community of Practice
DC = Data Controller
IRC = Independent Review Committee
LR - Lead Researcher
PHSREC = NSW Population & Health Services Research Ethics Committee
Your frequently asked questions
There is no limit to the number of active sub-studies within one research group. However, there may be a limit to the number of users who can access the secure research environment simultaneously to analyse the data. It is the Lead Researcher’s responsibility to decide how many sub-studies are submitted for approval and are active at any one time.
Only those who have been nominated as requiring access to the CanDLe datasets on the sub-study protocol will have access to the unit record data. Approval for data access user logins will be dependent on the approved sub-study protocol.
The Lead Researcher and research team will propose how long a sub-study will run in the protocol; this will need to be approved by ethics. However, access to secure research environment (e.g. SURE) may require ongoing funding. The Lead Researcher must ensure that there is funding available to cover the costs of accessing the secure research environment for this timeframe.
Additionally, progress reporting to ethics is a condition of ongoing approval. A Lead Researcher must submit an annual progress report to the CanDLe secretariat at CINSW-CanDLeProgram@health.nsw.gov.au by 31 December each year. The secretariat will submit to ethics on the Lead Researcher’s behalf.
Established Researcher: A researcher with at least five years’ experience.
Early Career Researcher: A researcher with no more than five years’ experience.
PhD Student: A researcher who is currently enrolled in a PhD and this project will contribute to it.
Master Student: A researcher who is currently enrolled in a Masters and this project will contribute to it.
Other Student: A researcher who is currently enrolled in another program such as Honours and this project will contribute to it.
No. A researcher will only use one login across multiple projects within that research team’s workspace.
Yes. Access to the secure research environment such as SURE are granted following approval of a sub-study protocol, not the Expression of Interest application. All researchers requiring access to datasets must be listed on the sub-study protocol.