Joining the CanDLe program
Research teams may apply to join the CanDLe program by submitting an Expression of Interest that nominates a Lead Researcher and Data Controller.
Applications will be assessed against the strict selection criteria by the CanDLe Independent Review Committee. The roles and responsibilities for the Lead Researcher and Data Controller are described in the CanDLe user protocol.
Lead Researcher Approval Process
Expression of Interest Rounds
There are two Expression of Interest rounds annually, where researchers can apply to be a Lead Researcher on the CanDLe program.
Note: these dates are indicative and may be subject to change.
| Opens | Closes (1:00 pm) | |
|---|---|---|
| CanDLe Lead Researcher Expression of Interest (Round 8) | 2 February 2024 | 13 March 2024 |
| CanDLe Lead Researcher Expression of Interest (Round 9) | 22 April 2024 | 5 June 2024 |
Submitting an Expression of Interest
Research group leaders who would like to participate in the CanDLe program as a Lead Researcher must submit the following documents to: CINSW-CanDLeProgram@health.nsw.gov.au before 1:00 pm on the closing date:
- Expression of Interest application (.docx)
- CanDLe Data Controller Nomination Form (.docx)
- Institution Endorsement Letter (.docx)
Applications will be reviewed by the CanDLe Independent Review Committee who provide recommendations to the Co-ordinating Principal Investigator to consider submission to ethics.
Lead Researcher Selection Criteria
- Expertise in analysing and interpreting linked health data (preferentially in cancer research), including a proven track record and high levels of research productivity.
- Experience or training handling potentially sensitive personal information or re-identifiable data.
- Experience supervising a research team that has undertaken analyses using large health administrative linked datasets.
- Justification of the key research themes/questions anticipated for analysis using CanDLe datasets.
- Endorsement of the Expression of Interest application by the applicant's Institution.
- Willingness and ability to participate in the Lead Researcher Community of Practice every six weeks.
Data Controller Selection Criteria
- Previous experience and relevant training in managing and working with large health administrative linked datasets.
- Understanding of and willingness to adhere to the Five Safes Framework.
- Willingness and ability to participate in the Data Controller’s Forum every six weeks.
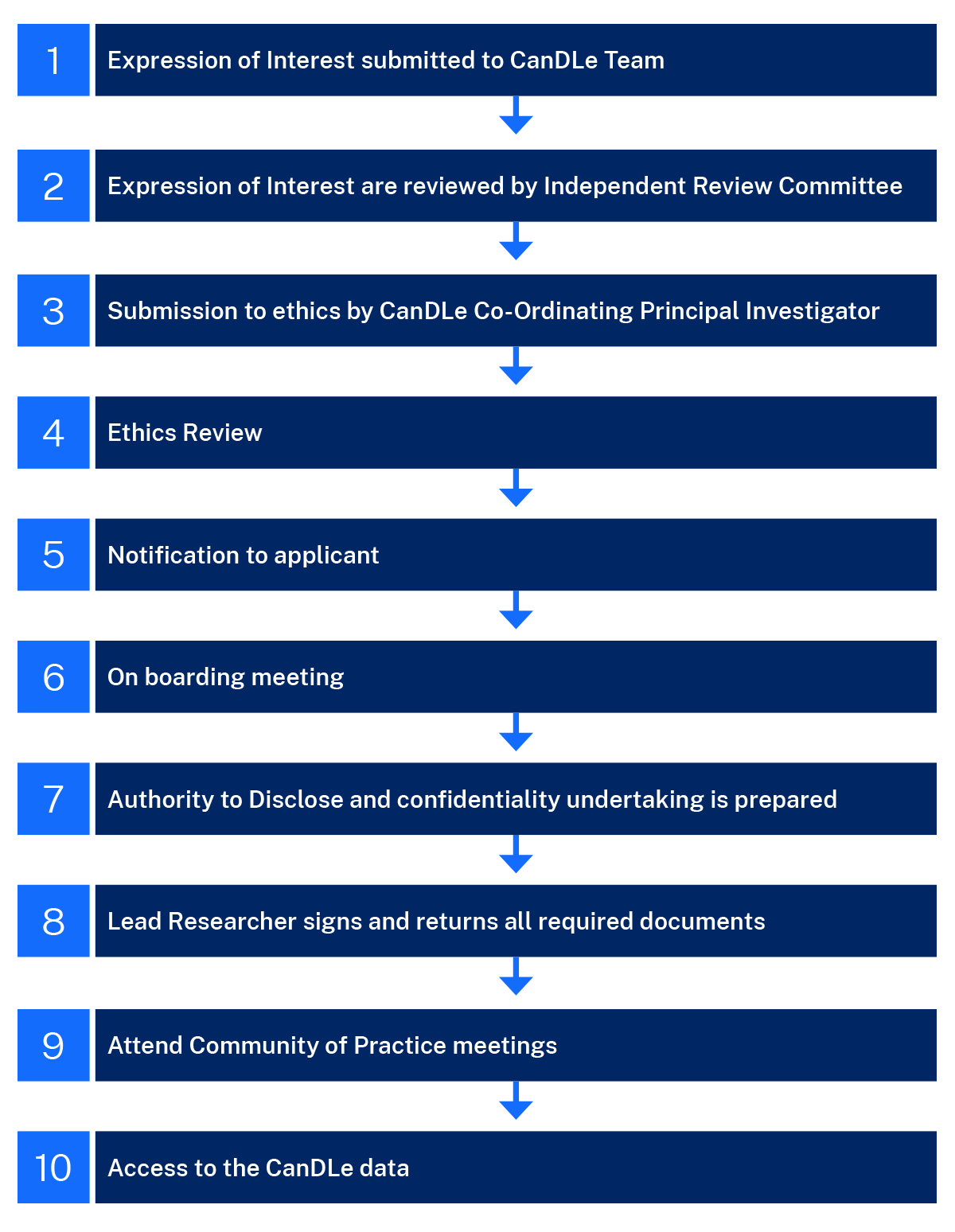
Lead Researcher approval process
- Expression of Interest submitted to CanDLe Team
Submissions to be emailed to: CINSW-CanDLeProgram@health.nsw.gov.au - Expression of Interest are reviewed by Independent Review Committee
Submissions are recommended or not recommended for review by the Co-Ordinating Principal Investigator - Submission to ethics by CanDLe Co-Ordinating Principal Investigator
The Co-Ordinating Principal Investigator will review the submission and if recommended, submit to the ethics committee for review. - Ethics Review
The ethics committee will review the submission and notify the CanDLe team of the outcome.
- Notification to applicant
The CanDLe team will notify the applicant of the ethics outcome. If successful, the CanDLe team will send the Lead Researcher appointment letter, Community of Practice Terms of Reference, and CanDLe User Protocol. - On-boarding meeting
The CanDLe team will meet the Lead Researcher and Data Controller. The Lead Researcher will confirm who will sign the Authority to Disclose Confidentiality Undertaking on behalf of their Institution. - Authority to Disclose and confidentiality undertaking is prepared
The Centre for Epidemiology and Evidence will sign the Authority to Disclose Unit Record Data Letter and Disclosure of Information. The CanDLe team will send to the Institution’s delegate. The Delegate must sign the Confidentiality Undertaking. - Lead Researcher signs and returns all required documents
The Lead Researcher must sign the following documents:
- Code of Conduct for Committees and Subcommittees
- Interest Declaration and Undertaking Form
- Data Access Declaration
- Secure Environment Service Order Form.
The Institution’s delegate must sign the Authority to Disclose Confidentiality Undertaking. - Attend Community of Practice meetings
Lead Researchers are expected to attend the meetings held every 6 weeks. Lead Researchers submit sub-study protocols for review by the Community of Practice. - Access to the CanDLe data
Access to data is granted following the return of all required documents, the set-up of a secure environment space, and the approval of a sub-study protocol.
Your frequently asked questions
Members of the research team that are anticipated to be involved in the majority of sub-studies conducted. A Key Research Team member may or may not require access to the datasets.
For example, an experienced clinician may assist in the development, planning and interpretation of a sub-study, but not require access to datasets. A biostatistician would likely assist in the development of the protocol and require access to datasets.
Changes in membership of the research team should be notified to the CanDLe team immediately via the change in research team form. Changes should also be noted in the annual progress report (.docx) which is due 31 December.
